Abstract
Introduction
Myelofibrosis (MF), is a myeloproliferative BCR-ABL1-negative neoplasm which is commonly associated with bone marrow failure, malignant transformation and death.
The median survival is estimated at 6 years, but it can range from months to many years.
Allogenic hematopoietic stem cell transplantation (alloSCT) is the only potentially curative therapy today available for high risk MF.
For patients relapsing after alloSCT, donor lymphocyte infusion (DLI) or second transplant have been described with variable results.
These strategies rely on the graft versus myelofibrosis (GVM) effect to eradicate the malignant clone. Herein, the clinical features and outcome of patients with MF relapsing after alloSCT treated at Policlinico San Martino are reported.
Methods
This retrospective analysis included all adult MF patients, age 18 years or older, who had undergone first alloSCT between 2011 to 2014 and subsequently relapsed.
Patients were treated on transplantation protocols that were available during the different time periods. Collected data included patient and disease characteristics at diagnosis and at transplant time, source of stem cells, incidence of acute GVHD, median duration of post-transplantation remission, details of salvage therapy and its response (table 1).
Relapse was diagnosed in presence of abnormal peripheral blood counts, declining donor bone marrow chimerism, increasing peripheral blood CD34 counts or blasts, and/or mutated JAK2 (if present before transplantation)
Early relapse was defined when occurring within day +180, whereas late relapse was identified when happening after day +180.
DLI or other strategies were administered at the onset of relapse as defined above.
Results
Twelve patients with relapsed MF were identified; patients with late and early relapse were 7 (58%) and 5 (42%) respectively.
The median age at transplant was 55 years old (range 31 - 57).
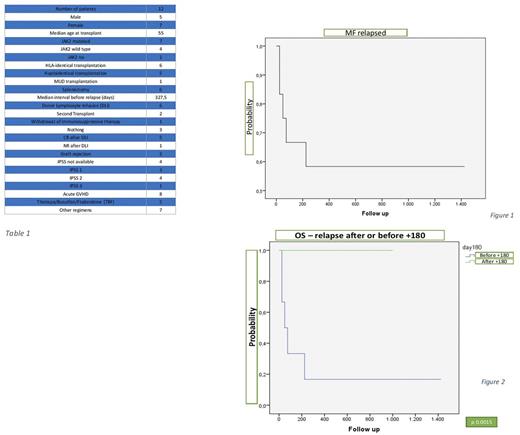
Median interval from transplant to relapse was 327,5 days (range 28 - 2058) and overall survival (OS) at 3 years was 58% (figure 1).
There was no significant statistical correlation between OS and type of transplant, IPSS, JAK2 status, splenomegaly, or development of GVHD.
Eight of the 12 relapsed patients (66%) had developed acute GVHD with a median time from transplant to the onset of 17 days; three of them developed also early relapse and died.
DLI were used in 6 patients, obtaining 5 complete response (CR) and 1 non-response (NR); the latter developed subsequent graft rejection and is in hematologic CR and his/her bone marrow (BM) evaluation showed absence of MF clone and fibrosis.
A patient transplanted with matched-unrelated donor (MUD) developed late relapse and was treated with withdrawal of immune suppressive therapy obtaining graft rejection; the BM evaluation showed absence of MF clone and fibrosis.
Two patients underwent to second transplants with success; now they are alive and in CR.
The remaining three patients didn't receive any therapy due the presence of active infections and very poor performance status.
All the late relapse patients are alive, while only one patient with early relapse is alive at the time of the analysis.
The OS at 3 years of the patients with late and early relapse was 100% and 18% respectively (figure 2).
Discussion and conclusions
With our results, a prolonged GVM seems to overlap with the definition of late relapse, as demonstrated that all late relapse patients are alive and with a good peripheral blood count.
DLI may be interpreted as a reinforcement of GVM effect enabling the eradication of residual disease as happened in 2 late relapse cases.
On the opposite side, early relapse may be characterized with an insufficient time to establish an efficacious GVM.
In conclusion, the OS in MF relapsed after alloSCT may depend on the time of relapse and consequently on GVM effect which may either reduce or eradicate the MF clone.
Angelucci: Roche: Other: Advisory board: biosimilars; Bluebird Bio: Other: Advisory board: Gene therapy in Thalassemia; Jazz: Other: Advisory board: AML; Novartis Oncology: Other: Protocol Telesto: sterring committee Chair; Celgene: Other: protocoll ACE 536 B-Thal 001: DMC Chair; Novartis Oncology Swiss: Other: Invited speakers sponsored satellite meeting during ; Celgene: Honoraria, Other: Advisory: research project ; Novartis Oncology: Other: Advisory board: iron toxicity.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal